An oxygen atom has a mass of 2.66 atomic mass units, a fundamental property that underpins its chemical and physical behavior. Understanding the mass of an oxygen atom is crucial in various scientific disciplines, from chemistry to medicine. This article delves into the concept of atomic mass, explores the factors influencing the mass of an oxygen atom, and discusses the measurement techniques and applications of this knowledge.
The mass of an oxygen atom is not a fixed value but can vary depending on the presence of different isotopes. Isotopes are atoms of the same element with the same atomic number but different neutron counts, resulting in varying masses.
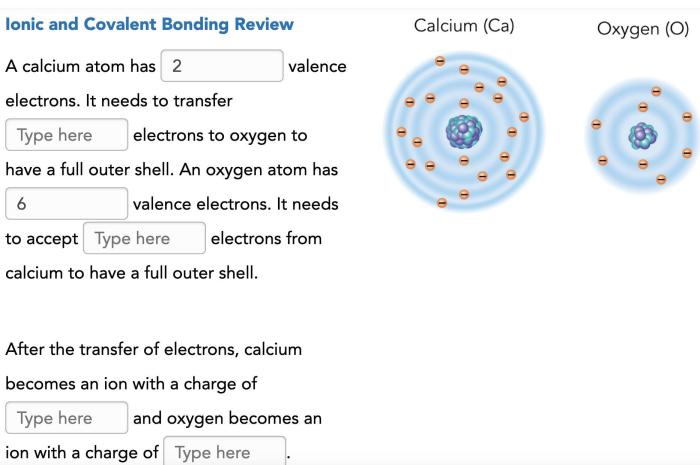
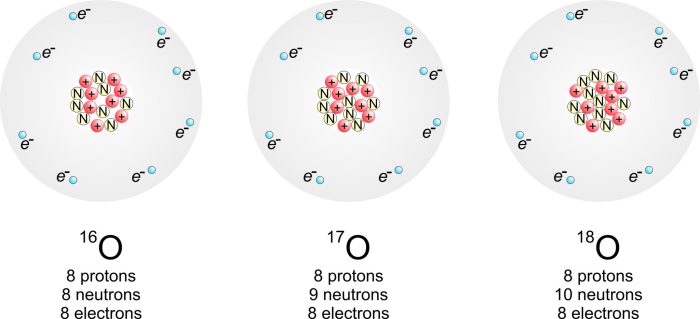
Oxygen has three naturally occurring isotopes: oxygen-16, oxygen-17, and oxygen-18. The most abundant isotope, oxygen-16, has eight protons and eight neutrons, contributing significantly to the average mass of an oxygen atom.
Oxygen Atom’s Mass
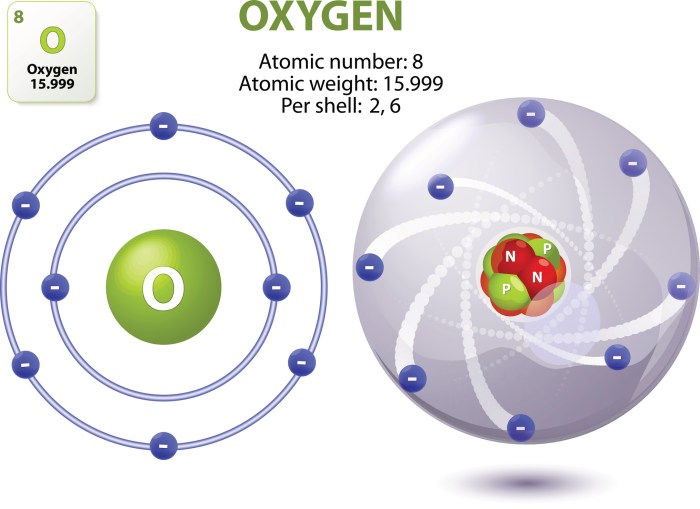
Atomic mass, expressed in atomic mass units (amu), represents the average mass of an atom of an element. It takes into account the contributions of all isotopes, which are variations of an element with different numbers of neutrons. The atomic mass of oxygen is 16.00 amu.
This value is significantly higher than that of hydrogen (1.008 amu), but lower than that of carbon (12.01 amu) and nitrogen (14.01 amu). The mass of an oxygen atom is primarily influenced by the number of protons and neutrons it contains, with neutrons contributing more to the overall mass due to their larger size.
Isotopes of Oxygen
Oxygen has three naturally occurring isotopes: 16O, 17O, and 18O. 16O is the most abundant isotope, accounting for approximately 99.76% of all oxygen atoms. 17O and 18O are less common, with abundances of 0.04% and 0.20%, respectively.
These isotopes differ in their neutron count. 16O has 8 neutrons, 17O has 9 neutrons, and 18O has 10 neutrons. The different neutron counts result in slightly different atomic masses: 15.994915 amu for 16O, 16.999132 amu for 17O, and 17.999161 amu for 18O.
Measurement of Oxygen Mass
The mass of an oxygen atom can be measured using various analytical techniques, including mass spectrometry. Mass spectrometers separate ions based on their mass-to-charge ratio. By measuring the relative abundance of different ions, the isotopic composition and average atomic mass of oxygen can be determined.
Other methods for measuring oxygen mass include nuclear magnetic resonance (NMR) spectroscopy and atomic absorption spectroscopy. These techniques provide complementary information and can be used to confirm the accuracy of mass spectrometry measurements.
Applications of Oxygen Mass, An oxygen atom has a mass of 2.66
Knowing the mass of an oxygen atom is crucial in various scientific fields, including chemistry, physics, and biology. It is used to determine the molecular weights of compounds, calculate reaction stoichiometry, and understand the behavior of oxygen in different environments.
In medicine, the mass of oxygen is important for understanding the uptake and utilization of oxygen by cells. It is also used to calibrate medical equipment, such as oxygen sensors and ventilators.
FAQ Insights: An Oxygen Atom Has A Mass Of 2.66
What is the significance of knowing the mass of an oxygen atom?
The mass of an oxygen atom is crucial for determining the molecular weights of compounds, understanding chemical reactions, and predicting the physical properties of substances.
How are the masses of different oxygen isotopes measured?
Mass spectrometry is a technique commonly used to measure the masses of different oxygen isotopes. It involves separating ions based on their mass-to-charge ratio, allowing for precise determination of isotopic masses.
What are the applications of oxygen mass spectrometry?
Oxygen mass spectrometry finds applications in various fields, including environmental monitoring (e.g., detecting pollutants), medical diagnostics (e.g., identifying metabolic disorders), and materials science (e.g., analyzing isotopic ratios in geological samples).