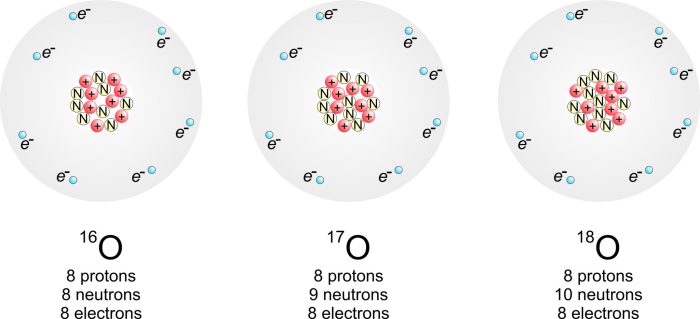
Chemistry Semester 2 Exam Review Answers provides a comprehensive review of the fundamental concepts, problem-solving techniques, and applications of chemistry covered in the second semester of a typical undergraduate chemistry course. This guide is designed to help students prepare for their final exams and succeed in their chemistry studies.
This guide covers a wide range of topics, including the periodic table, chemical reactions, thermochemistry, kinetics and equilibrium, organic chemistry, and electrochemistry. Each topic is explained in a clear and concise manner, with numerous examples and practice problems to help students understand the concepts and apply them to real-world situations.
Concepts and Principles
Chemistry is the study of matter and its properties. It is a fundamental science that has applications in many fields, including medicine, engineering, and materials science.
The periodic table is a tabular arrangement of the chemical elements, organized by their atomic number, electron configuration, and recurring chemical properties. It is a powerful tool for understanding the chemical behavior of elements and for predicting the properties of new elements.
Chemical reactions are processes that involve the rearrangement of atoms and molecules. There are many different types of chemical reactions, including combustion, synthesis, decomposition, and exchange reactions. Chemical reactions are used in a wide variety of applications, including the production of food, fuels, and pharmaceuticals.
Problem-Solving Techniques
Problem-solving is an essential skill in chemistry. There are a number of strategies that can be used to solve chemistry problems, including dimensional analysis and equilibrium calculations.
Dimensional analysis is a technique that uses the units of measurement to convert between different units. This technique can be used to solve a variety of chemistry problems, such as converting between grams and moles.
Equilibrium calculations are used to determine the concentrations of reactants and products at equilibrium. Equilibrium is a state of balance in which the forward and reverse reactions are occurring at the same rate. Equilibrium calculations are used in a variety of applications, such as predicting the solubility of a compound.
Thermochemistry, Chemistry semester 2 exam review answers
Thermochemistry is the study of heat and its relationship to chemical reactions. Enthalpy is a measure of the heat content of a system. Entropy is a measure of the disorder of a system. Free energy is a measure of the spontaneity of a reaction.
Hess’s Law is a law that states that the enthalpy change of a reaction is equal to the sum of the enthalpy changes of the individual steps of the reaction. Hess’s Law can be used to calculate the enthalpy change of a reaction even if the reaction cannot be carried out in one step.
Kinetics and Equilibrium
Chemical kinetics is the study of the rates of chemical reactions. The rate of a reaction is determined by a number of factors, including the temperature, the concentration of the reactants, and the presence of a catalyst.
Chemical equilibrium is a state of balance in which the forward and reverse reactions are occurring at the same rate. The equilibrium constant is a measure of the relative concentrations of the reactants and products at equilibrium.
Organic Chemistry
Organic chemistry is the study of compounds that contain carbon. Organic compounds are found in all living things and are used in a wide variety of products, including fuels, plastics, and pharmaceuticals.
The basic principles of organic chemistry include the study of functional groups, molecular structures, and reaction mechanisms.
Electrochemistry
Electrochemistry is the study of the relationship between electricity and chemical reactions. Redox reactions are reactions that involve the transfer of electrons. Electrochemical cells are devices that use redox reactions to generate electricity or to drive chemical reactions.
Electrochemistry is used in a variety of applications, including batteries, fuel cells, and corrosion prevention.
FAQ Corner: Chemistry Semester 2 Exam Review Answers
What topics are covered in Chemistry Semester 2 Exam Review Answers?
Chemistry Semester 2 Exam Review Answers covers a wide range of topics, including the periodic table, chemical reactions, thermochemistry, kinetics and equilibrium, organic chemistry, and electrochemistry.
Is Chemistry Semester 2 Exam Review Answers suitable for students of all levels?
Chemistry Semester 2 Exam Review Answers is designed for undergraduate students who have completed the first semester of a typical chemistry course. However, it may also be useful for high school students who are preparing for AP Chemistry or other advanced chemistry courses.
Can Chemistry Semester 2 Exam Review Answers help me prepare for my final exam?
Yes, Chemistry Semester 2 Exam Review Answers is an excellent resource for preparing for your final exam. It provides a comprehensive review of the essential concepts and includes numerous practice problems to help you test your understanding.